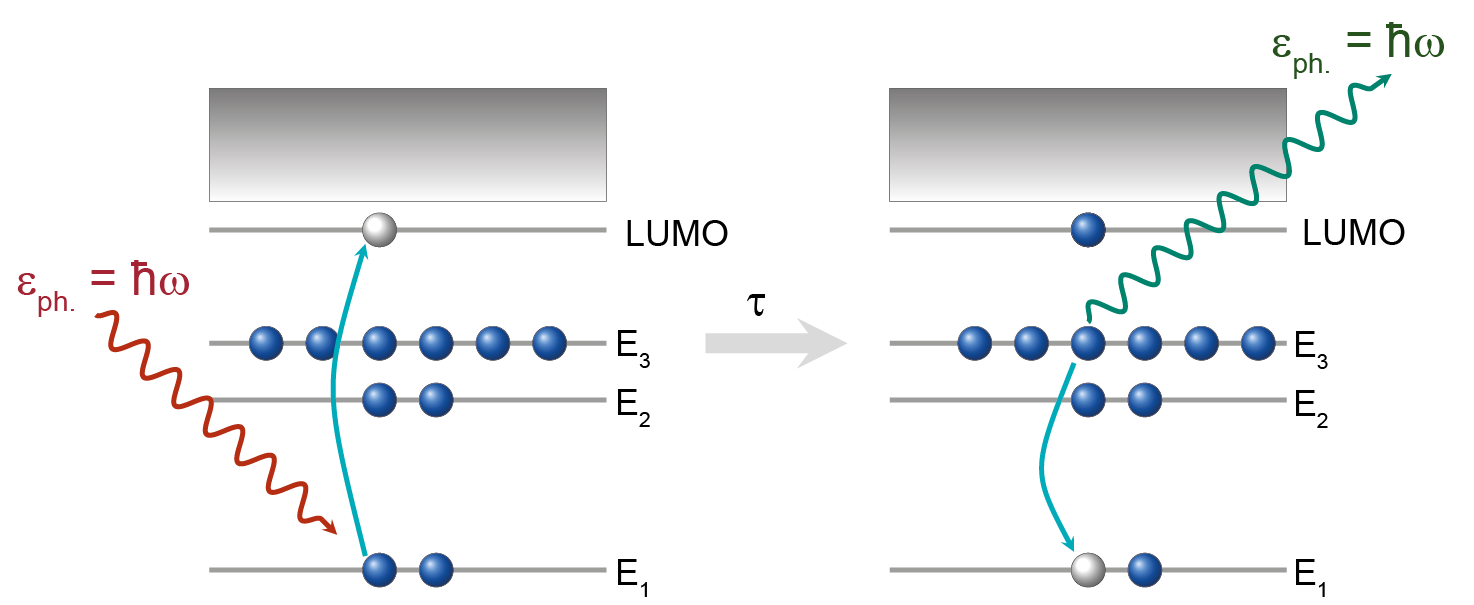
Resonant X-ray Emission Spectroscopy (RXES) - Resonant Inelastic X-ray Scattering (RIXS)
1. Sub-femtosecond nuclear dynamics
Resonant X-ray Emission spectroscopy is very suitable to investigate in detail dynamical processes in molecules on a femtoseconds (1fs = 10−15 s) time scale of or even subfemtoseconds. Because nuclear motions in a molecular system are usually associated with timescales of a few femtoseconds, while molecular vibrations and rotations typically occur on time scales of the order of 10−13 to 10−12 s, Resonant X-ray Emission spectroscopy (also known as Resonant Inelastic X-Ray Scattering (RIXS)) is very suitable to investigate in detail dynamical processes in molecules. The investigation of molecular dynamics on such ultrafast time scales can be obtained by using the so-called ‘internal core-hole clock’ where the characteristic time is given by the lifetime of the core-excited state. The development of 3rd generation synchrotron light sources in last decades has allowed experiments RIXS in the tender x-ray regime (2–10 keV), which has only recently become available for atomic and molecular spectroscopy studies because of high flux/high resolution provided by synchrotron beamlines yielding unprecedented insight into nuclear dynamics.
1. Sub-femtosecond nuclear dynamics
We have then demonstrated that RIXS allows to monitor nuclear dynamics on a sub-femtosecond timescale and to obtain, with help of theory, information on potential energy surfaces of the states involved in the diffusion process. ( M. Simon et al. Phys. Rev. A 73, 020706(R) (2006) , L. Journel et al. Rev. Sci. Instrum. 80, 093105 (2009), L. El Khoury et al., J. Chem. Phys. 136 024319 (2012), T. Marchenko et al, J. Chem. Phys. 134 144308 (2011).

2. Chemical sensitivity
This spectroscopy also has a chemical selectivity allowing to gain information on the local environment of the probed atom in the molecular system. In particular, effects of the nature of the ligand on the emission spectra X have been highlighted. A pioneer paper (D.W. Lindle et al., Phys. Rev. Lett. 60, 1010 (1988)) has demonstrated that RIXS from randomly oriented gas-phase molecules can display large degrees of polarization. In this case, a Cl 1s core electron from CH3Cl was excited into the anti-bonding LUMO orbital oriented along the C-Cl bond axis. The core-excited state decays rapidly via x-ray emission involving occupied valence orbitals (oriented parallel or perpendicular to the C-Cl bond). The characteristic time for the emission process is given by the core-hole lifetime of the intermediate core-excited state (~1 fs for Cl K hole). Thus the polarization of the scattered x-rays reflects the ‘alignment’ due to the absorption process in the first step between orbitals of given symmetry. This paper was the first demonstration of the technique of polarized-RIXS where RIXS spectra are recorded at various angles between incident and scattered photon polarizations.

More recent results have demonstrated that polarized RIXS is a spectroscopy very sensitive to the chemical environment and a new probe of molecular-field effects on electronic structure. (R. Guillemin et al., Phys. Rev. Lett. 101, 133003 (2008), S. Carniato et al., Phys. Rev. A 80, 032513 (2009), R. Guillemin et al., Phys. Rev. A 86, 013407 (2012)). RIXS from several chlorine containing molecules in which chlorine atoms have different chemical environments have been measured. 1s2p RIXS after excitation of a 1s into the anti-bonding LUMO involves Cl 2p3/2 and 2p1/2 outer-core-electrons. Measurements of polarized RIXS spectra allow direct and very sensitive determinations of Cl 2pz and 2pxy atomic orbital populations within the 2p3/2 and 2p1/2 molecular spin-orbit states. The results exhibit a linear dichroic signal, Cl 2p3/2:2p1/2 spin-orbit ratios measured on-resonance varies as a function of the polarization angle. For each molecule, the ratios differ significantly from the statistical atomic-2p ratio of 2:1 indicating that molecular spin-orbit state are very sensitive to the chemical environment.
This result is completely general and has been observed in a variety of chlorinated systems (HCl, CH3Cl, CF3Cl, and Cl2). Recently, it has been demonstrated that determination of Cl 2p atomic-orbital populations could be used as a sensitive measure of the electronegativity of the species bound to the chlorine atom and an electronegativity scale may be built as it has be shown that Cl 1s polarized RIXS is an effective way to probe charge sharing between a Cl atom and any species bound to it (S. Carniato et al., J. Chem. Phys. 137, 144303 (2012)).
3. Interference effects
Moreover, using the large lifetime broadening, we have been able to observe in the HCl system that several electronic states can be excited coherently. The radiative decay to the same final state via different intermediate states reveals strong quantum interferences which has been observed for the first time via resonant XX-ray emission (M. Kavcic et al., Phys. Rev. Lett. 105, 113004 (2010)).

4. Thomson scattering
In case of the HCl molecule, we have measured polarization resolved elastic peak as a function of the incident photon energy. We have observed interferences between Thomson and Resonant elastic scattering depending on the polarization. This effect, if predicted theoretically, was never been observed before (S. Carniato et al., J. Chem. Phys. 137, 094311 (2012)).

5. MOSARIX
